Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamamoto, Yusuke*; Watanabe, Takahiro; Niwa, Masakazu; Shimada, Koji
JAEA-Testing 2023-003, 67 Pages, 2024/02
A long-term geosphere stability for geological disposal is evaluated by the past geological environmental changes and modern conditions. Stable hydrogen and oxygen isotope ratios ( D,
D, 
 O) of geological samples are useful information to estimate the past environmental changes and modern conditions. Recently, the thermal conversion elemental analyzer and isotope ratio mass spectrometer (TC-EA/IRMS) were installed in the Tono Geoscience Center for
O) of geological samples are useful information to estimate the past environmental changes and modern conditions. Recently, the thermal conversion elemental analyzer and isotope ratio mass spectrometer (TC-EA/IRMS) were installed in the Tono Geoscience Center for  D and
D and 
 O measurements of geological samples. In this study, we reported analytical methods of
O measurements of geological samples. In this study, we reported analytical methods of  D and
D and 
 O using international standard reference materials. In addition, evaluation tests of uncertainty by repeated analyses of the standards were performed using the TC-EA/IRMS. Furthermore, the
O using international standard reference materials. In addition, evaluation tests of uncertainty by repeated analyses of the standards were performed using the TC-EA/IRMS. Furthermore, the  D and
D and 
 O analyses by the TC- EA/IRMS were also applied to fault rock samples.
O analyses by the TC- EA/IRMS were also applied to fault rock samples.
Iwasa, Toma; Arima, Tatsumi*
JAEA-Technology 2021-036, 23 Pages, 2022/03
Knowledge on the liquefaction (thermal decomposition and melting) temperatures of MA-bearing nitride fuels for transmutation by accelerator-driven system is essential for elucidation of the fuel behaviors under abnormal condition and for the safety analysis. A melting temperature measurement system for refractory materials was developed based on a laser spot heating method, which is expected to measure in a very short time with a small amount of sample, and demonstration tests using refractory metals and zirconium nitride were performed. As the results, it was found that this melting temperature measurement system can be applicable up to the temperatures around 3000 K which is close to the thermal decomposition temperature of nitride fuels and we confirmed the technical feasibility of this system for future application to small specimens of transuranium nitrides.
Kawaguchi, Munemichi
Proceedings of 2020 International Conference on Nuclear Engineering (ICONE 2020) (Internet), 6 Pages, 2020/08
In decommissioning sodium-cooled fast reactors, the operators can be exposed to radiation during dismantling of the cold trap equipment (C/T). The C/T has higher dose equipment because the C/T trapped the tritium of the fission product during the operation to purify the sodium coolant. In this research, the thermal decomposition temperature and rate of sodium hydride were measured as the fundamental research for improvement of the thermorlysis method prior to the dismantling. We measured the thermal decomposition temperature and rate using sodium hydride powder (95.3%, Sigma-Aldrich) in Al O
O crucible with TG-DTA (STA2500, NETASCH Japan). The heating rates were set to
crucible with TG-DTA (STA2500, NETASCH Japan). The heating rates were set to  = 2.0, 5.0, 10.0, 20.0 K/min, and the weight decrease was measured. The thermal decomposition temperature and rate were obtained from the temperature of the onset of the weigh decrease and the Kissinger plot, respectively. Furthermore, we set to the thermal decomposition temperature of around 600 K, and the weight decreasing rates were measured. The change of the chemical composition of the sodium hydride with heating (from NaH to Na) was measured with X-Ray Diffraction (XRD) analysis. As a result, the thermal decomposition occurred at around 600 K, and the almost all hydrogen in the sodium hydride released within 1 h. The thermal decomposition rate strongly depended on the heating temperature.
= 2.0, 5.0, 10.0, 20.0 K/min, and the weight decrease was measured. The thermal decomposition temperature and rate were obtained from the temperature of the onset of the weigh decrease and the Kissinger plot, respectively. Furthermore, we set to the thermal decomposition temperature of around 600 K, and the weight decreasing rates were measured. The change of the chemical composition of the sodium hydride with heating (from NaH to Na) was measured with X-Ray Diffraction (XRD) analysis. As a result, the thermal decomposition occurred at around 600 K, and the almost all hydrogen in the sodium hydride released within 1 h. The thermal decomposition rate strongly depended on the heating temperature.
 C thin film
C thin filmNorimatsu, Wataru*; Matsuda, Keita*; Terasawa, Tomoo; Takata, Nao*; Masumori, Atsushi*; Ito, Keita*; Oda, Koji*; Ito, Takahiro*; Endo, Akira*; Funahashi, Ryoji*; et al.
Nanotechnology, 31(14), p.145711_1 - 145711_7, 2020/04
Times Cited Count:6 Percentile:38.95(Nanoscience & Nanotechnology)We show that boron-doped epitaxial graphene can be successfully grown by thermal decomposition of a boron carbide thin film, which can also be epitaxially grown on a silicon carbide substrate. The interfaces of B C on SiC and graphene on B
C on SiC and graphene on B C had a fixed orientation relation, having a local stable structure with no dangling bonds. The first carbon layer on B
C had a fixed orientation relation, having a local stable structure with no dangling bonds. The first carbon layer on B C acts as a buffer layer, and the overlaying carbon layers are graphene. Graphene on B
C acts as a buffer layer, and the overlaying carbon layers are graphene. Graphene on B C was highly boron doped, and the hole concentration could be controlled over a wide range of 2
C was highly boron doped, and the hole concentration could be controlled over a wide range of 2 10
10 to 2
to 2 10
10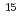 cm
cm . Highly boron-doped graphene exhibited a spin-glass behavior, which suggests the presence of local antiferromagnetic ordering in the spin-frustration system. Thermal decomposition of carbides holds the promise of being a technique to obtain a new class of wafer-scale functional epitaxial graphene for various applications.
. Highly boron-doped graphene exhibited a spin-glass behavior, which suggests the presence of local antiferromagnetic ordering in the spin-frustration system. Thermal decomposition of carbides holds the promise of being a technique to obtain a new class of wafer-scale functional epitaxial graphene for various applications.
Inagaki, Yoshiyuki; Sakaba, Nariaki
Shokubai, 61(2), p.92 - 96, 2019/04
The outline of the membrane IS process to produce hydrogen by thermochemical water splitting using solar heat at around 650 C is described. The membrane technology has been applied to the three main reaction of the IS process to lower the reaction temperature and reduce the amount of circulation materials in the process. The key component technologies such as catalysts, membranes and corrosion resistant materials have been developed. The study was supported in part by the Council for Science, Technology and Innovation, Cross-ministerial Strategic Innovation Promotion Program, "Energy Carrier".
C is described. The membrane technology has been applied to the three main reaction of the IS process to lower the reaction temperature and reduce the amount of circulation materials in the process. The key component technologies such as catalysts, membranes and corrosion resistant materials have been developed. The study was supported in part by the Council for Science, Technology and Innovation, Cross-ministerial Strategic Innovation Promotion Program, "Energy Carrier".
Abe, Hitoshi; Tashiro, Shinsuke; Miyoshi, Yoshinori
Nihon Genshiryoku Gakkai Wabun Rombunshi, 6(1), p.10 - 21, 2007/03
In MOX fuel fabrication facility, zinc stearate will be added into the MOX powder as addition material. If the material is added in large excess by miss operation, criticality characteristics of the MOX fuel would be influenced because it has neutron moderation effect. If criticality condition should be induced by the excess addition, physical variations, such as melting and pyrolysis of the material, must be caused by the fission energy and dynamic characteristics of the MOX fuel must be affected. To contribute quantitative evaluation of the dynamic characteristics, thermal properties data such as exo/endothermic calorific values, reaction rates, etc. with the respective physical variations and release behavior of pyrolysis gas were measured. It was found the exo/endothermic behavior with rinsing temperature of the material could be divided into six regions and rapid pressure rise caused by the pyrolysis reaction over about 400  C. Furthermore, on the basis of the results, evaluation model for the thermal properties under the criticality condition was also investigated.
C. Furthermore, on the basis of the results, evaluation model for the thermal properties under the criticality condition was also investigated.
Tomimitsu, Hiroshi; Hasegawa, Yuji*; Aizawa, Kazuya
Physics Letters A, 309(3-4), p.183 - 188, 2003/03
Times Cited Count:0 Percentile:0.01(Physics, Multidisciplinary)no abstracts in English
Kato, Chiaki; Yano, Masaya*; Kiuchi, Kiyoshi; Sugimoto, Katsuhisa*
Corrosion Engineering, 52(1), p.53 - 67, 2003/01
The effects of heat-transfer on the corrosion of zirconium was examined in boiling nitric acid solutions with various concentrations. Corrosion mass losses and electrochemical polarization curves were measured on the heat-transfer and isothermal surfaces in the solutions. It was found that the corrosion rate of zirconium was higher on the heat-transfer surface than that on the isothermal surface. The rate increased with increasing nitric acid concentration and solution temperature. The increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition on the surface and the removal of the decomposition product from solution by boiling bubbles. The redox potential of 12 mol/dm nitric acid on a boiling heat-transfer surface was very close to the breakdown potential of primary passivity of zirconium. This suggests the initiation of SCC on a boiling heat-transfer surface in a nuclear fuel reprocessing.
nitric acid on a boiling heat-transfer surface was very close to the breakdown potential of primary passivity of zirconium. This suggests the initiation of SCC on a boiling heat-transfer surface in a nuclear fuel reprocessing.
Kato, Chiaki; Kiuchi, Kiyoshi; Sugimoto, Katsuhisa*
Corrosion Engineering, 52(1), p.69 - 85, 2003/01
It is necessary to know the generation mechanism of high equilibrium potential in the solutions. Existing nitrogen oxides in nitric acid solutions were first analyzed by Raman spectroscopy and then existing amount of nitrogen oxides were examined by thermodynamic calculation using the SOLGASMIX software. The Raman spectroscopic analysis showed that the existing amount of un-dissociated HNO increased with increasing nitric acid concentration and solution temperature. The thermodynamic calculation showed that the important nitrogen oxides in nitric acid solutions are HNO
increased with increasing nitric acid concentration and solution temperature. The thermodynamic calculation showed that the important nitrogen oxides in nitric acid solutions are HNO , NO
, NO
 , HNO
, HNO , NO
, NO , and NO. The equilibrium potential of nitric acid solutions is, however, mainly decided by the HNO
, and NO. The equilibrium potential of nitric acid solutions is, however, mainly decided by the HNO /HNO
/HNO equilibrium. The thermodynamic calculation also suggested that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition of nitrous acid on the surface and the continuous removal of decomposition product from the solutions by boiling bubbles.
equilibrium. The thermodynamic calculation also suggested that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition of nitrous acid on the surface and the continuous removal of decomposition product from the solutions by boiling bubbles.
Kato, Chiaki; Yano, Masaya*; Kiuchi, Kiyoshi; Sugimoto, Katsuhisa*
Zairyo To Kankyo, 52(1), p.35 - 43, 2003/01
The effects of heat-transfer on the corrosion of zirconium was examined in boiling nitric acid solutions with various concentrations. Corrosion mass losses and electrochemical polarization curves were measured on the heat-transfer and isothermal surfaces in the solutions. It was found that the corrosion rate of zirconium was higher on the heat-transfer surface than that on the isothermal surface. The rate increased with increasing nitric acid concentration and solution temperature. The increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition on the surface and the removal of the decomposition product from solution by boiling bubbles. The redox potential of 12 mol/dm3 nitric acid on a boiling heat-transfer surface was very close to the breakdown potential of primary passivity of zirconium. This suggests the initiation of SCC on a boiling heat-transfer surface in a nuclear fuel reprocessing.
Kato, Chiaki; Kiuchi, Kiyoshi; Sugimoto, Katsuhisa*
Zairyo To Kankyo, 52(1), p.44 - 52, 2003/01
In order to understand corrosion of metals in nitric acid solutions, it is necessary to know the generation mechanism of high equilibrium potential in the solutions, especially under boiling conditions. The Raman spectroscopic analysis showed that the existing amount of un-dissociated HNO increased with increasing nitric acid concentration and solution temperature. The existing amount of NO
increased with increasing nitric acid concentration and solution temperature. The existing amount of NO also increased by thermal decomposition. The thermodynamic calculation showed that the important nitrogen oxides in nitric acid solutions are HNO
also increased by thermal decomposition. The thermodynamic calculation showed that the important nitrogen oxides in nitric acid solutions are HNO , NO
, NO , HNO
, HNO , NO
, NO , and NO. The equilibrium potential of nitric acid solutions is, however, mainly decided by the HNO
, and NO. The equilibrium potential of nitric acid solutions is, however, mainly decided by the HNO /HNO
/HNO equilibrium. The thermodynamic calculation also suggested that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition of nitrous acid on the surface and the continuous removal of decomposition product from the solutions by boiling bubbles.
equilibrium. The thermodynamic calculation also suggested that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition of nitrous acid on the surface and the continuous removal of decomposition product from the solutions by boiling bubbles.
Yamauchi, Toshihiko
Kankyo Kagakkai-Shi, 14(6), p.567 - 575, 2001/12
no abstracts in English
Tsukamoto, Michio; Takada, Junichi; Koike, Tadao; Watanabe, Koji*; Miyata, Teijiro*; Nishio, Gunji*; Murata, Mikio*; Uchiyama, Gunzo
JAERI-Tech 2001-031, 47 Pages, 2001/03
no abstracts in English
 laser irradiation
laser irradiationYamauchi, Toshihiko; Kamei, Yasutaka*; Ito, Shinichi*; Furukawa, Yukio*; Minehara, Eisuke
Kankyo Kagakkai-Shi, 14(1), p.73 - 76, 2001/01
no abstracts in English
Makuuchi, Keizo
Porima Daijesuto, 52(1), p.89 - 108, 2000/01
no abstracts in English
Hasegawa, Shin; Takeshita, Hidefumi; Yoshii, Fumio; Makuuchi, Keizo; Nishimoto, S.*
IAEA-TECDOC-1023, p.413 - 424, 1998/06
no abstracts in English
; Aoyama, Makoto; ; Yamanouchi, Takamichi
PNC TN8410 97-319, 143 Pages, 1997/10
The fire and explosion incident occurred at Bituminization Demonstration Facility of PNC Tokai Works on March 11, 1997. In order to ascertain the cause of incident, the investigation has been pushed forward. During investigation, we obtained essential information from operators, such as softness of bituminized product, white smoke generated from bituminized product. This condition has never been observed comparing past normal operation. Therefore, we assumed that temperature of bituminized product had increased more than expected. In order to confirm above assumption, we made experiment for obtaining the relationship between temperature and fluidity of bituminized product. Simulated bituminized product was heated up to each temperature (210, 230, 250, 270 C) in a pot and poured down into an another pot. We observed the fluidity of bituminized product when it flowing down into a pot. The fluidity of bituminized product increased with high temperature. The fluidity of bituminized product at 270
C) in a pot and poured down into an another pot. We observed the fluidity of bituminized product when it flowing down into a pot. The fluidity of bituminized product increased with high temperature. The fluidity of bituminized product at 270 C looked similar to fluidity of bituminized product that had ignited itself at the incident. White smoke generated from bituminized product and amounts of white smoke increased with high temperature. The smoke was considered to be gas that generated through thermal decomposition or volatilization of bitumen.
C looked similar to fluidity of bituminized product that had ignited itself at the incident. White smoke generated from bituminized product and amounts of white smoke increased with high temperature. The smoke was considered to be gas that generated through thermal decomposition or volatilization of bitumen.
Kihara, Shinji; Yahata, Taneaki; *; ; *; ; ; *
JAERI-Research 97-037, 43 Pages, 1997/05
no abstracts in English